Vertebrate
| Vertebrate | |
|---|---|

| |
| Diversity of vertebrates: Acipenser oxyrinchus (Actinopterygii), an African bush elephant (Tetrapoda), a tiger shark (Chondrichthyes) and a river lamprey (Agnatha). | |
| Scientific classification | |
| Domain: | Eukaryota |
| Kingdom: | Animalia |
| Superphylum: | Deuterostomia |
| Phylum: | Chordata |
| Clade: | Olfactores |
| Subphylum: | Vertebrata J-B. Lamarck, 1801[2] |
| Infraphyla | |
| Synonyms | |
|
Ossea Batsch, 1788[2] | |
Vertebrates (/ˈvɜːrtəbrɪts, -ˌbreɪts/)[3] are deuterostomal animals with bony or cartilaginous axial endoskeleton — known as the vertebral column, spine or backbone — around and along the spinal cord, including all fish, amphibians, reptiles, birds and mammals. The vertebrates consist of all the taxa within the subphylum Vertebrata (/ˌvɜːrtəˈbreɪtə/)[4] and represent the overwhelming majority of the phylum Chordata, with currently about 69,963 species described.[5]
Vertebrates comprise groups such as the following infraphyla and classes:
- Agnatha or jawless fish, which include:
- †Conodonta
- †Ostracodermi
- Cyclostomi (hagfish and lampreys)
- Gnathostomata or jawed vertebrates, which include:
- †Placodermi
- †Acanthodii
- Chondrichthyes or cartilaginous fish, (sharks, rays and ratfish)
- Osteichthyes or bony fish, which include:
- Actinopterygii or ray-finned fish, which comprises the majority of living bony fish and over half of all living vertebrates, including:
- Cladistia (bichirs and relatives)
- Chondrostei (sturgeons and paddlefish)
- Holostei (bowfins and gars)
- Teleostei (96% of living fish species)
- Sarcopterygii or lobe-finned fish, which include:
- Actinistia (coelacanths)
- Dipnomorpha (lungfish)
- Tetrapoda[a] or limbed vertebrates
- Amphibians (lissamphibians, as well as the extinct temnospondyls and lepospondyls)
- Amniotes or true land vertebrates
- Sauropsids (reptiles and birds, as well as the extinct parareptiles, dinosaurs, pterosaurs and Mesozoic marine reptiles)
- Synapsids (mammals as well as all their extinct relatives and pelycosaurid/therapsid ancestors)
- Actinopterygii or ray-finned fish, which comprises the majority of living bony fish and over half of all living vertebrates, including:
Extant vertebrates vary in body lengths ranging from the frog species Paedophryne amauensis, at as little as 7.7 mm (0.30 in), to the blue whale, at up to 33 m (108 ft). Vertebrates make up less than five percent of all described animal species; the rest are described as invertebrates, an informal paraphyletic group comprising all that lack vertebral columns, which include non-vertebrate chordates such as lancelets.
The vertebrates traditionally include the hagfish, which do not have proper vertebrae due to their loss in evolution,[7] though their closest living relatives, the lampreys, do.[8] Hagfish do, however, possess a cranium. For this reason, the vertebrate subphylum is sometimes referred to as Craniata or "craniates" when discussing morphology. Molecular analysis since 1992 has suggested that hagfish are most closely related to lampreys,[9] and so also are vertebrates in a monophyletic sense. Others consider them a sister group of vertebrates in the common taxon of Craniata.[10]
Etymology
[edit]The word vertebrate derives from the Latin word vertebratus (Pliny), meaning joint of the spine.[11] A similarly derived word is vertebra, which refers to any of the irregular bones or segments of the spinal column.[12]
Anatomy and morphology
[edit]All vertebrates are built along the basic chordate body plan of five synapomorphies:
- A rigid axial endoskeleton (vertebral column) developed around an elastic notochord (which becomes the intervertebral discs), running dorsally to the gut tube (hence the common name for the vertebral column, the "backbone") along the length of the animal.[13]
- The axial endoskeleton typically continues beyond the anus to form an elongated post-anal tail,[14] although some vertebrates evolved to become tailless with only a vestigial coccyx.
- A dorsal nerve cord, which folds and fuses into a hollow neural tube during embryonic development and eventually gives rise to the brain and spinal cord, runs more dorsally to the axial endoskeleton (enclosed by protective skeletal extensions known as neural arches), with a fore-end enlargement that is contained within a distinct skeletonized braincase (hence the alternative name for vertebrates, the craniates).
- All vertebrates possess pharyngeal arches, which develops into the gill arches, jaw, hyoid and/or the middle ear ossicles.
- An iodine-concentrating organ called the endostyle, which function as a filter feeding organ in basal jawless vertebrates, has evolved into the thyroid in most adult vertebrates.
Vertebral column
[edit]
With only one exception, the defining characteristic of all vertebrates is the vertebral column, in which the embryonic notochord found in all chordates is replaced by a segmented series of mineralized elements called vertebrae separated by fibrocartilaginous intervertebral discs, which are embryonic and evolutionary remnants of the notochord. Hagfish are the only extant vertebrate whose notochord persists and is not integrated/ replaced by the vertebral column. A few vertebrates have secondarily lost this feature and retain the notochord into adulthood, such as the sturgeon[15] and coelacanth. Jawed vertebrates are typified by paired appendages (fins or limbs, which may be secondarily lost), but this trait is not required to qualify an animal as a vertebrate.
The vertebral column is the central component of the axial skeleton, which structurally supports the core body segments and unpaired appendages such as tail and sails. Together with the appendicular skeleta that support paired appendages (particularly limbs), this forms an internal skeletal system, i.e. the endoskeleton, which is vastly different to the exoskeleton and hydroskeleton ubiquitously seen in invertebrates. The endoskeleton structure enables a more concentrated layout of skeletal tissues, with soft tissues attaching outside (and thus not restricted by the volume of) the skeleton, which allows vertebrates to achieve much larger body sizes than invertebrates of the same skeletal mass.
Gills
[edit]
Most vertebrates are aquatic and carry out gas exchange via gills. The gills are carried right behind the head, bordering the posterior margins of a series of crescentic openings from the pharynx to the outside. Each gill is supported by a cartilaginous or bony gill arch,[16] which develop embryonically from pharyngeal arches. Bony fish have three pairs of gill arches, cartilaginous fish have five to seven pairs, while the primitive jawless fish have seven pairs. The ancestral vertebrates likely had more arches than seven, as some of their chordate relatives have more than 50 pairs of gill opens,[14] although most, if not all, of these openings are actually involved in filter feeding rather than respiration. In jawed vertebrates, the first gill arch pair evolved into the jointed jaws and form an additional oral cavity ahead of the pharynx. Research also suggests that the sixth branchial arch contributed to the formation of the vertebrate shoulder, which separated the head as a distinct part of the body.[17]
In amphibians and some primitive bony fishes, the larvae bear external gills, branching off from the gill arches.[18] These are reduced in adulthood, their respiratory function taken over by the internal gills proper in fishes and by cutaneous respiration in most amphibians. While some amphibians such as axolotl retain the external gills into adulthood, the complex internal gill system as seen in fish apparently being irrevocably lost very early in the evolution of tetrapods, who evolved lungs (which are homologous to swim bladders) to breathe air.[19]
While the more specialized terrestrial vertebrates lack gills, the gill arches form during fetal development, and form the basis of essential structures such as jaws, the thyroid gland, the larynx, the columella (corresponding to the stapes in mammals) and, in mammals, the malleus and incus.[14]
Central nervous system
[edit]The central nervous system of vertebrates is based on the embryonic dorsal nerve cord (which then flattens into a neural plate before folding and fusing over into a hollow neural tube) running along the dorsal aspect of the notochord. Of particular importance and unique to vertebrates is the presence of neural crest cells, which are progenitor cells critical to coordinating the functions of cellular components.[20] Neural crest cells migrate through the body from the dorsal nerve cord during development, initiate the formation of neuronal ganglia and various special sense organs.[21][22][23] The peripheral nervous system forms when neural crest cells branch out laterally from the dorsal nerve cord and migrate together with the mesodermal somites to innervate the various different structures that develop in the body.
The vertebrates are the only chordate group with neural cephalization, and their neural functions are centralized towards a series of enlarged clusters in the head, which give rise to a brain. A slight swelling of the anterior end of the nerve cord is found in invertebrate chordates such as lancelets (a sister subphylum known as the cephalochordates), though it lacks eyes and other complex special sense organs comparable to those of vertebrates. Other chordates do not show any trends towards cephalization.[14]
The rostral end of the neural tube is expanded by a thickening of the walls and expansion of the central canal of spinal cord into three primary brain vesicles: the prosencephalon (forebrain), mesencephalon (midbrain) and rhombencephalon (hindbrain), which are further differentiated in the various vertebrate groups.[24] Two laterally placed retinas and optical nerves form around outgrowths from the midbrain, except in hagfish, though this may be a secondary loss.[25][26] The forebrain is more well-developed in most tetrapods and subdivided into the telencephalon and diencephalon, while the midbrain dominates in fish and some salamanders. In vertebrates with paired appendages, especially tetrapods, a pair of secondary enlargements of the hindbrain become the cerebella, which modulate complex motor coordinations. The brain vesicles are usually bilaterally symmetrical, giving rise to the paired cerebral hemispheres in mammals.[24]
The resultant anatomy of a central nervous system arising from a single nerve cord dorsal to the gut tube, headed by a series of (typically paired) brain vesicles, is unique to vertebrates. This is in stark contrast to invertebrates with well-developed central nervous systems such as arthropods and cephalopods, who have an often ladder-like ventral nerve cord made of paired segmental ganglia on the opposite (ventral) side of the gut tube, with a split brain stem circumventing the foregut around each side to form a brain on the dorsal side of the mouth.[14] The higher functions of the vertebrate CNS are highly centralized towards the brain (particularly the forebrain), while the invertebrate CNS is significantly more decentralized with the segmental ganglia having substantial neural autonomy independent of the brain (which itself is a fused cluster of segmental ganglia from the rostral metameres).
Another distinct neural feature of vertebrates is the axonal/dendritic myelination in both central (via oligodendrocytes) and peripheral nerves (via neurolemmocytes). Although myelin insulation is not unique to vertebrates — many annelids and arthropods also have myelin sheath formed by glia cells, with the kuruma shrimp having twice the conduction velocity of any vertebrates — vertebrate myelination is annular and non-fenestrated, and the combination of myelination and encephalization have given vertebrates a unique advantage in developing higher neural functions such as complex motor coordination and cognition. It also allows vertebrates to evolve larger sizes while still maintaining considerable body reactivity, speed and agility (in contrast, invertebrates typically become sensorily slower and motorically clumsier with larger sizes), which are crucial for the eventual adaptive success of vertebrates in seizing dominant niches of higher trophic levels in both terrestrial and aquatic ecosystems.
Molecular signatures
[edit]In addition to the morphological characteristics used to define vertebrates (i.e. the presence of a notochord, the development of a vertebral column from the notochord, a dorsal nerve cord, pharyngeal gills, a post-anal tail, etc.), molecular markers known as conserved signature indels (CSIs) in protein sequences have been identified and provide distinguishing criteria for the subphylum Vertebrata.[27] Specifically, 5 CSIs in the following proteins: protein synthesis elongation factor-2 (EF-2), eukaryotic translation initiation factor 3 (eIF3), adenosine kinase (AdK) and a protein related to ubiquitin carboxyl-terminal hydrolase are exclusively shared by all vertebrates and reliably distinguish them from all other metazoan.[27] The CSIs in these protein sequences are predicted to have important functionality in vertebrates.
A specific relationship between vertebrates and tunicates is also strongly supported by two CSIs found in the proteins Rrp44 (associated with exosome complex) and serine palmitoyltransferase, that are exclusively shared by species from these two subphyla but not cephalochordates, indicating vertebrates are more closely related to tunicates than cephalochordates.[27]
Evolutionary history
[edit]External relationships
[edit]Originally, the "Notochordata hypothesis" suggested that the Cephalochordata is the sister taxon to Craniata (Vertebrata). This group, called the Notochordata, was placed as sister group to the Tunicata (Urochordata). Although this was once the leading hypothesis,[28] studies since 2006 analyzing large sequencing datasets strongly support Olfactores (tunicates + vertebrates) as a monophyletic clade,[29][30][27] and the placement of Cephalochordata as sister-group to Olfactores (known as the "Olfactores hypothesis"). As chordates, they all share the presence of a notochord, at least during a stage of their life cycle.
The following cladogram summarizes the systematic relationships between the Olfactores (vertebrates and tunicates) and the Cephalochordata.
| Chordata |
| ||||||||||||
First vertebrates
[edit]
Vertebrates originated during the Cambrian explosion, which saw a rise in organism diversity. The earliest known vertebrates belongs to the Chengjiang biota[31] and lived about 518 million years ago.[1] These include Haikouichthys, Myllokunmingia,[31] Zhongjianichthys,[32] and probably Haikouella.[33] Unlike the other fauna that dominated the Cambrian, these groups had the basic vertebrate body plan: a notochord, rudimentary vertebrae, and a well-defined head and tail.[34] All of these early vertebrates lacked jaws in the common sense and relied on filter feeding close to the seabed.[35][page needed] A vertebrate group of uncertain phylogeny, small eel-like conodonts, are known from microfossils of their paired tooth segments from the late Cambrian to the end of the Triassic.[36]
From fish to amphibians
[edit]
The first jawed vertebrates may have appeared in the late Ordovician (~445 mya) and became common in the Devonian period, often known as the "Age of Fishes".[37] The two groups of bony fishes, the Actinopterygii and Sarcopterygii, evolved and became common.[38] The Devonian also saw the demise of virtually all jawless fishes save for lampreys and hagfish, as well as the Placodermi, a group of armoured fish that dominated the entirety of that period since the late Silurian as well as the eurypterids, dominant animals of the preceding Silurian, and the anomalocarids. By the middle of the Devonian, several droughts, anoxic events and oceanic competition lead a lineage of sarcopterygii to leave water, eventually establishing themselves as terrestrial tetrapods in the succeeding Carboniferous.
Mesozoic vertebrates
[edit]Amniotes branched from amphibious tetrapods early in the Carboniferous period. The synapsid amniotes were dominant during the late Paleozoic, the Permian, while diapsid amniotes became dominant during the Mesozoic. In the sea, the teleosts and sharks became dominant. Mesothermic synapsids called cynodonts gave rise to endothermic mammals and diapsids called dinosaurs eventually gave rise to endothermic birds, both in the Jurassic.[39] After all dinosaurs except birds went extinct by the end of the Cretaceous, birds and mammals diversified and filled their niches.
After the Mesozoic
[edit]The Cenozoic world saw great diversification of bony fishes, amphibians, reptiles, birds and mammals.[40][41]
Over half of all living vertebrate species (about 32,000 species) are fish (non-tetrapod craniates), a diverse set of lineages that inhabit all the world's aquatic ecosystems, from the Tibetan stone loach (Triplophysa stolickai) in western Tibetan hot springs near Longmu Lake at an elevation of 5,200 metres (17,100 feet) to an unknown species of snailfish (genus Pseudoliparis) in the Izu–Ogasawara Trench at a depth of 8,336 metres (27,349 feet).[42][43] Many fish varieties are the main predators in most of the world's freshwater and marine water bodies . The rest of the vertebrate species are tetrapods, a single lineage that includes amphibians (with roughly 7,000 species); mammals (with approximately 5,500 species); and reptiles and birds (with about 20,000 species divided evenly between the two classes). Tetrapods comprise the dominant megafauna of most terrestrial environments and also include many partially or fully aquatic groups (e.g., sea snakes, penguins, cetaceans).
Classification
[edit]There are several ways of classifying animals. Evolutionary systematics relies on anatomy, physiology and evolutionary history, which is determined through similarities in anatomy and, if possible, the genetics of organisms. Phylogenetic classification is based solely on phylogeny.[44] Evolutionary systematics gives an overview; phylogenetic systematics gives detail. The two systems are thus complementary rather than opposed.[45]
Traditional classification
[edit]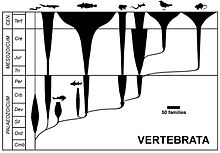
Conventional classification has living vertebrates grouped into seven classes based on traditional interpretations of gross anatomical and physiological traits. This classification is the one most commonly encountered in school textbooks, overviews, non-specialist, and popular works. The extant vertebrates are:[14]
- Subphylum Vertebrata
- Class Agnatha (jawless fishes)
- Class Chondrichthyes (cartilaginous fishes)
- Class Osteichthyes (bony fishes)
- Class Amphibia (amphibians)
- Class Reptilia (reptiles)
- Class Aves (birds)
- Class Mammalia (mammals)
In addition to these, there are two classes of extinct armoured fishes, the Placodermi and the Acanthodii, both considered paraphyletic.
Other ways of classifying the vertebrates have been devised, particularly with emphasis on the phylogeny of early amphibians and reptiles. An example based on Janvier (1981, 1997), Shu et al. (2003), and Benton (2004)[46] is given here († = extinct):

- Subphylum Vertebrata
- †Palaeospondylus
- Infraphylum Agnatha or Cephalaspidomorphi (lampreys and other jawless fishes)
- Superclass †Anaspidomorphi (anaspids and relatives)
- Infraphylum Gnathostomata (vertebrates with jaws)
- Class †Placodermi (extinct armoured fishes)
- Class Chondrichthyes (cartilaginous fishes)
- Class †Acanthodii (extinct spiny "sharks")
- Superclass Osteichthyes (bony fishes)
- Class Actinopterygii (ray-finned bony fishes)
- Class Sarcopterygii (lobe-finned fishes, including the tetrapods)
- Superclass Tetrapoda (four-limbed vertebrates)
- Class Amphibia (amphibians, some ancestral to the amniotes)—now a paraphyletic group
- Class Synapsida (mammals and the extinct mammal-like reptiles)
- Class Sauropsida (reptiles and birds)
While this traditional classification is orderly, most of the groups are paraphyletic, i.e. do not contain all descendants of the class's common ancestor.[46] For instance, descendants of the first reptiles include modern reptiles, mammals and birds; the agnathans have given rise to the jawed vertebrates; the bony fishes have given rise to the land vertebrates; the traditional "amphibians" have given rise to the reptiles (traditionally including the synapsids or mammal-like "reptiles"), which in turn have given rise to the mammals and birds. Most scientists working with vertebrates use a classification based purely on phylogeny,[47] organized by their known evolutionary history and sometimes disregarding the conventional interpretations of their anatomy and physiology.
Phylogenetic relationships
[edit]In phylogenetic taxonomy, the relationships between animals are not typically divided into ranks but illustrated as a nested "family tree" known as a phylogenetic tree. The cladogram below is based on studies compiled by Philippe Janvier and others for the Tree of Life Web Project and Delsuc et al.,[48][49] and complemented (based on,[50][51] and [52]). A dagger (†) denotes an extinct clade, whereas all other clades have living descendants.








| Vertebrata/ |
|
†"Ostracodermi" †"Placodermi" | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Craniata |
Note that, as shown in the cladogram above, the †"Ostracodermi" (armoured jawless fishes) and †"Placodermi" (armoured jawed fishes) are shown to be paraphylectic groups, separated from gnathostomes and eugnathostomes respectively.[53][54]
Also note that Teleostei (Neopterygii) and Tetrapoda (amphibians, mammals, reptiles, birds) each make up about 50% of today's vertebrate diversity, while all other groups are either extinct or rare. The next cladogram shows the extant clades of tetrapods (the four-limbed vertebrates), and a selection of extinct (†) groups:
Note that reptile-like amphibians, mammal-like reptiles, and non-avian dinosaurs are all paraphyletic.
The placement of hagfish on the vertebrate tree of life has been controversial. Their lack of proper vertebrae (among with other characteristics found in lampreys and jawed vertebrates) led phylogenetic analyses based on morphology to place them outside Vertebrata. Molecular data, however, indicates they are vertebrates closely related to lampreys. A study by Miyashita et al. (2019), 'reconciliated' the two types of analysis as it supports the Cyclostomata hypothesis using only morphological data.[55]
| |||||||
Number of extant species
[edit]The number of described vertebrate species are split between tetrapods and fish. The following table lists the number of described extant species for each vertebrate class as estimated in the IUCN Red List of Threatened Species, 2014.3.[56]
| Vertebrate groups | Image | Class | Estimated number of described species[56][57] |
Group totals[56] | ||
|---|---|---|---|---|---|---|
| Anamniote lack amniotic membrane so need to reproduce in water |
Jawless | Fish | 
|
Myxini (hagfish) |
78 | >32,900 |

|
Hyperoartia (lamprey) |
40 | ||||
| Jawed | 
|
cartilaginous fish |
>1,100 | |||

|
ray-finned fish |
>32,000 | ||||

|
lobe-finned fish |
8 | ||||
| Tetrapods | 
|
amphibians | 7,302 | 33,278 | ||
| Amniote have amniotic membrane adapted to reproducing on land |

|
reptiles | 10,711 | |||

|
mammals | 5,513 | ||||

|
birds | 10,425 | ||||
| Total described species | 66,178 | |||||
The IUCN estimates that 1,305,075 extant invertebrate species have been described,[56] which means that less than 5% of the described animal species in the world are vertebrates.
Vertebrate species databases
[edit]The following databases maintain (more or less) up-to-date lists of vertebrate species:
- Fish: Fishbase
- Amphibians: Amphibiaweb
- Reptiles: Reptile Database
- Birds: Avibase
- Mammals: Mammal species of the World
Reproductive systems
[edit]Nearly all vertebrates undergo sexual reproduction. They produce haploid gametes by meiosis. The smaller, motile gametes are spermatozoa and the larger, non-motile gametes are ova. These fuse by the process of fertilisation to form diploid zygotes, which develop into new individuals.
Inbreeding
[edit]During sexual reproduction, mating with a close relative (inbreeding) often leads to inbreeding depression. Inbreeding depression is considered to be largely due to expression of deleterious recessive mutations.[58] The effects of inbreeding have been studied in many vertebrate species.
In several species of fish, inbreeding was found to decrease reproductive success.[59][60][61]
Inbreeding was observed to increase juvenile mortality in 11 small animal species.[62]
A common breeding practice for pet dogs is mating between close relatives (e.g. between half- and full siblings).[63] This practice generally has a negative effect on measures of reproductive success, including decreased litter size and puppy survival.[64][65][66]
Incestuous matings in birds result in severe fitness costs due to inbreeding depression (e.g. reduction in hatchability of eggs and reduced progeny survival).[67][68][69]
Inbreeding avoidance
[edit]As a result of the negative fitness consequences of inbreeding, vertebrate species have evolved mechanisms to avoid inbreeding.
Numerous inbreeding avoidance mechanisms operating prior to mating have been described. Toads and many other amphibians display breeding site fidelity. Individuals that return to natal ponds to breed will likely encounter siblings as potential mates. Although incest is possible, Bufo americanus siblings rarely mate.[70] These toads likely recognize and actively avoid close kin as mates. Advertisement vocalizations by males appear to serve as cues by which females recognize their kin.[70]
Inbreeding avoidance mechanisms can also operate subsequent to copulation. In guppies, a post-copulatory mechanism of inbreeding avoidance occurs based on competition between sperm of rival males for achieving fertilization.[71] In competitions between sperm from an unrelated male and from a full sibling male, a significant bias in paternity towards the unrelated male was observed.[71]
When female sand lizards mate with two or more males, sperm competition within the female's reproductive tract may occur. Active selection of sperm by females appears to occur in a manner that enhances female fitness.[72] On the basis of this selective process, the sperm of males that are more distantly related to the female are preferentially used for fertilization, rather than the sperm of close relatives.[72] This preference may enhance the fitness of progeny by reducing inbreeding depression.
Outcrossing
[edit]Mating with unrelated or distantly related members of the same species is generally thought to provide the advantage of masking deleterious recessive mutations in progeny[73] (see heterosis). Vertebrates have evolved numerous diverse mechanisms for avoiding close inbreeding and promoting outcrossing[74] (see inbreeding avoidance).
Outcrossing as a way of avoiding inbreeding depression has been especially well studied in birds. For instance, inbreeding depression occurs in the great tit (Parus major) when the offspring are produced as a result of a mating between close relatives. In natural populations of the great tit, inbreeding is avoided by dispersal of individuals from their birthplace, which reduces the chance of mating with a close relative.[75]
Purple-crowned fairywren females paired with related males may undertake extra-pair matings that can reduce the negative effects of inbreeding, despite ecological and demographic constraints.[69]
Southern pied babblers (Turdoides bicolor) appear to avoid inbreeding in two ways: through dispersal and by avoiding familiar group members as mates.[76] Although both genders disperse locally, they move outside the range where genetically related individuals are likely to be encountered. Within their group, individuals only acquire breeding positions when the opposite-sex breeder is unrelated.
Cooperative breeding in birds typically occurs when offspring, usually males, delay dispersal from their natal group in order to remain with the family to help rear younger kin.[77] Female offspring rarely stay at home, dispersing over distances that allow them to breed independently or to join unrelated groups.
Parthenogenesis
[edit]Parthenogenesis is a natural form of reproduction in which growth and development of embryos occur without fertilization.
Reproduction in squamate reptiles is ordinarily sexual, with males having a ZZ pair of sex determining chromosomes, and females a ZW pair. However, various species, including the Colombian Rainbow boa (Epicrates maurus), Agkistrodon contortrix (copperhead snake) and Agkistrodon piscivorus (cotton mouth snake) can also reproduce by facultative parthenogenesis—that is, they are capable of switching from a sexual mode of reproduction to an asexual mode—resulting in production of WW female progeny.[78][79] The WW females are likely produced by terminal automixis.[citation needed]
Mole salamanders are an ancient (2.4–3.8 million year-old) unisexual vertebrate lineage.[80] In the polyploid unisexual mole salamander females, a premeiotic endomitotic event doubles the number of chromosomes. As a result, the mature eggs produced subsequent to the two meiotic divisions have the same ploidy as the somatic cells of the female salamander. Synapsis and recombination during meiotic prophase I in these unisexual females is thought to ordinarily occur between identical sister chromosomes and occasionally between homologous chromosomes. Thus little, if any, genetic variation is produced. Recombination between homeologous chromosomes occurs only rarely, if at all.[81] Since production of genetic variation is weak, at best, it is unlikely to provide a benefit sufficient to account for the long-term maintenance of meiosis in these organisms.[citation needed]
Self-fertilization
[edit]Two killifish species, the mangrove killifish (Kryptolebias marmoratus) and Kryptolebias hermaphroditus, are the only known vertebrates to self-fertilize.[82] They produce eggs and sperm by meiosis and routinely reproduce by self-fertilisation. This capacity has apparently persisted for at least several hundred thousand years.[83] Each individual hermaphrodite normally fertilizes itself through uniting inside the fish's body of an egg and a sperm that it has produced by an internal organ.[84] In nature, this mode of reproduction can yield highly homozygous lines composed of individuals so genetically uniform as to be, in effect, identical to one another.[85][86] Although inbreeding, especially in the extreme form of self-fertilization, is ordinarily regarded as detrimental because it leads to expression of deleterious recessive alleles, self-fertilization does provide the benefit of fertilization assurance (reproductive assurance) at each generation.[85]
Population trends
[edit]The Living Planet Index, following 16,704 populations of 4,005 species of vertebrates, shows a decline of 60% between 1970 and 2014.[87] Since 1970, freshwater species declined 83%, and tropical populations in South and Central America declined 89%.[88] The authors note that, "An average trend in population change is not an average of total numbers of animals lost."[88] According to WWF, this could lead to a sixth major extinction event.[89] The five main causes of biodiversity loss are land-use change, overexploitation of natural resources, climate change, pollution and invasive species.[90]
See also
[edit]- Marine vertebrate – Marine animals with a vertebrate column
- Invertebrate
- Endoskeleton
- Skeletal system of the horse
- Taxonomy of the vertebrates (Young, 1962) – Classification of spine-possessing animals according to some authorities
Notes
[edit]- ^ Tetrapoda are cladistically included within Sarcopterygii.[6]
References
[edit]- ^ a b Yang, Chuan; Li, Xian-Hua; Zhu, Maoyan; Condon, Daniel J.; Chen, Junyuan (2018). "Geochronological constraint on the Cambrian Chengjiang biota, South China" (PDF). Journal of the Geological Society. 175 (4): 659–666. Bibcode:2018JGSoc.175..659Y. doi:10.1144/jgs2017-103. ISSN 0016-7649. S2CID 135091168. Archived (PDF) from the original on 9 October 2022.
- ^ a b Nielsen, C. (July 2012). "The authorship of higher chordate taxa". Zoologica Scripta. 41 (4): 435–436. doi:10.1111/j.1463-6409.2012.00536.x. S2CID 83266247.
- ^ "vertebrate". Dictionary.com Unabridged (Online). n.d.
- ^ "Vertebrata". Dictionary.com Unabridged (Online). n.d.
- ^ "Table 1a: Number of species evaluated in relation to the overall number of described species, and numbers of threatened species by major groups of organisms". IUCN Red List. 18 July 2019.
- ^ Ahlberg, Per Erik (2021). "A comparative genomic framework for the fish-tetrapod transition". Science China Life Sciences. 64 (4): 664–666. doi:10.1007/s11427-021-1903-x. PMID 33660224.
- ^ Ota, Kinya G.; Fujimoto, Satoko; Oisi, Yasuhiro; Kuratani, Shigeru (25 January 2017). "Identification of vertebra-like elements and their possible differentiation from sclerotomes in the hagfish". Nature Communications. 2: 373. Bibcode:2011NatCo...2..373O. doi:10.1038/ncomms1355. ISSN 2041-1723. PMC 3157150. PMID 21712821.
- ^ Kuraku; et al. (December 1999). "Monophyly of Lampreys and Hagfishes Supported by Nuclear DNA–Coded Genes". Journal of Molecular Evolution. 49 (6): 729–35. Bibcode:1999JMolE..49..729K. doi:10.1007/PL00006595. PMID 10594174. S2CID 5613153.
- ^ Stock, D.; Whitt, G. S. (7 August 1992). "Evidence from 18S ribosomal RNA sequences that lampreys and hagfish form a natural group". Science. 257 (5071): 787–789. Bibcode:1992Sci...257..787S. doi:10.1126/science.1496398. PMID 1496398.
- ^ Nicholls, H. (10 September 2009). "Mouth to Mouth". Nature. 461 (7261): 164–166. doi:10.1038/461164a. PMID 19741680.
- ^ "vertebrate". Online Etymology Dictionary. Dictionary.com.
- ^ "vertebra". Online Etymology Dictionary. Dictionary.com.
- ^ Waggoner, Ben. "Vertebrates: More on Morphology". UCMP. Retrieved 13 July 2011.
- ^ a b c d e f Romer, A.S. (1949): The Vertebrate Body. W.B. Saunders, Philadelphia. (2nd ed. 1955; 3rd ed. 1962; 4th ed. 1970)
- ^ Liem, K. F.; Walker, W. F. (2001). Functional anatomy of the vertebrates: an evolutionary perspective. Harcourt College Publishers. p. 277. ISBN 978-0-03-022369-3.
- ^ Scott, T. (1996). Concise encyclopedia biology. Walter de Gruyter. p. 542. ISBN 978-3-11-010661-9.
- ^ Brazeau, Martin D.; Castiello, Marco; El Fassi El Fehri, Amin; Hamilton, Louis; Ivanov, Alexander O.; Johanson, Zerina; Friedman, Matt (20 November 2023). "Fossil evidence for a pharyngeal origin of the vertebrate pectoral girdle". Nature. 623 (7987): 550–554. Bibcode:2023Natur.623..550B. doi:10.1038/s41586-023-06702-4. hdl:10044/1/107350. PMC 10651482. PMID 37914937.
- ^ Szarski, Henryk (1957). "The Origin of the Larva and Metamorphosis in Amphibia". The American Naturalist. 91 (860): 283–301. doi:10.1086/281990. JSTOR 2458911. S2CID 85231736.
- ^ Clack, J. A. (2002): Gaining ground: the origin and evolution of tetrapods. Indiana University Press, Bloomington, Indiana. 369 pp
- ^ Teng, Lu; Labosky, Patricia A. (2006). "Neural Crest Stem Cells". Neural Crest Induction and Differentiation. Advances in Experimental Medicine and Biology. Vol. 589. pp. 206–212. doi:10.1007/978-0-387-46954-6_13. ISBN 978-0-387-35136-0. ISSN 0065-2598. PMID 17076284.
- ^ Gans, C.; Northcutt, R. G. (1983). "Neural crest and the origin of vertebrates: a new head". Science. 220 (4594): 268–273. Bibcode:1983Sci...220..268G. doi:10.1126/science.220.4594.268. PMID 17732898. S2CID 39290007.
- ^ Bronner, M. E.; LeDouarin, N. M. (1 June 2012). "Evolution and development of the neural crest: An overview". Developmental Biology. 366 (1): 2–9. doi:10.1016/j.ydbio.2011.12.042. PMC 3351559. PMID 22230617.
- ^ Dupin, E.; Creuzet, S.; Le Douarin, N.M. (2007) "The Contribution of the Neural Crest to the Vertebrate Body". In: Jean-Pierre Saint-Jeannet, Neural Crest Induction and Differentiation, pp. 96–119, Springer Science & Business Media. ISBN 9780387469546. doi:10.1007/978-0-387-46954-6_6. Full text
- ^ a b Hildebrand, M.; Gonslow, G. (2001): Analysis of Vertebrate Structure. 5th edition. John Wiley & Sons, Inc. New York
- ^ "Keeping an eye on evolution". PhysOrg.com. 3 December 2007. Retrieved 4 December 2007.
- ^ "Hyperotreti". tolweb.org.
- ^ a b c d Gupta, Radhey S. (January 2016). "Molecular signatures that are distinctive characteristics of the vertebrates and chordates and supporting a grouping of vertebrates with the tunicates". Molecular Phylogenetics and Evolution. 94 (Pt A): 383–391. Bibcode:2016MolPE..94..383G. doi:10.1016/j.ympev.2015.09.019. ISSN 1055-7903. PMID 26419477.
- ^ Stach, Thomas (2008). "Chordate phylogeny and evolution: a not so simple three-taxon problem". Journal of Zoology. 276 (2): 117–141. doi:10.1111/j.1469-7998.2008.00497.x.
- ^ Delsuc, F (2006). "Tunicates and not cephalochordates are the closest living relatives of vertebrates" (PDF). Nature. 439 (7079): 965–968. Bibcode:2006Natur.439..965D. doi:10.1038/nature04336. PMID 16495997. S2CID 4382758. Archived (PDF) from the original on 9 October 2022.
- ^ Dunn, C.W. (2008). "Broad phylogenetic sampling improves resolution of the animal tree of life". Nature. 452 (7188): 745–749. Bibcode:2008Natur.452..745D. doi:10.1038/nature06614. PMID 18322464. S2CID 4397099.
- ^ a b Shu, D-G.; Luo, H-L.; Conway Morris, S.; Zhang, X-L.; Hu, S-X.; Chen, L.; Han, J.; Zhu, M.; Li, Y.; Chen, L-Z. (1999). "Lower Cambrian vertebrates from south China". Nature. 402 (6757): 42–46. Bibcode:1999Natur.402...42S. doi:10.1038/46965. ISSN 0028-0836. S2CID 4402854.
- ^ Shu, D. (2003). "A paleontological perspective of vertebrate origin". Chinese Science Bulletin. 48 (8): 725–735. doi:10.1360/03wd0026.
- ^ Chen, J.-Y.; Huang, D.-Y.; Li, C.-W. (1999). "An early Cambrian craniate-like chordate". Nature. 402 (6761): 518–522. Bibcode:1999Natur.402..518C. doi:10.1038/990080. S2CID 24895681.
- ^ Waggoner, B. "Vertebrates: Fossil Record". UCMP. Archived from the original on 29 June 2011. Retrieved 15 July 2011.
- ^ Tim Haines, T.; Chambers, P. (2005). The Complete Guide to Prehistoric Life. Firefly Books.
- ^ Donoghue, P. C. J.; Forey, P. L.; Aldridge, R. J. (May 2000). "Conodont affinity and chordate phylogeny". Biological Reviews. 75 (2): 191–251. doi:10.1111/j.1469-185X.1999.tb00045.x. PMID 10881388. S2CID 22803015.
- ^ Encyclopædia Britannica: a new survey of universal knowledge, Volume 17. Encyclopædia Britannica. 1954. p. 107.
- ^ Berg, L. R.; Solomon, E. P.; Martin, D. W. (2004). Biology. Cengage Learning. p. 599. ISBN 978-0-534-49276-2.
- ^ Cloudsley-Thompson, J. L. (2005). Ecology and behaviour of Mesozoic reptiles. Springer. p. 6. ISBN 9783540224211.
- ^ Pires, Mathias; Mankin, Brian; Silvestro, Daniele; Quental, Tiago (26 September 2018). "Diversification dynamics of mammalian clades during the K–Pg mass extinction". Biology Letters. 14 (9). doi:10.1098/rsbl.2018.0458. PMC 6170748. PMID 30258031.
- ^ Lowery, Christopher; Fraass, Andrew (8 April 2019). "Morphospace expansion paces taxonomic diversification after end Cretaceous mass extinction". Nature Ecology & Evolution. 3 (6): 900–904. Bibcode:2019NatEE...3..900L. doi:10.1038/s41559-019-0835-0. hdl:1983/fb08c3c1-c203-4780-bc90-5994ec1030ff. PMID 30962557. S2CID 102354122 – via Nature.
- ^ Kottelat, M. (2012). "Conspectus_cobitidum.pdf Conspectus cobitidum: an inventory of the loaches of the world (Teleostei: Cypriniformes: Cobitoidei)" (PDF). The Raffles Bulletin of Zoology. Supplement No. 26: 1–199.
- ^ "Scientists find deepest fish ever recorded at 8,300 metres underwater near Japan". The Guardian. 3 April 2023. Retrieved 6 July 2024.
- ^ Andersen, N. M.; Weir, T. A. (2004). Australian water bugs: their biology and identification (Hemiptera-Heteroptera, Gerromorpha & Nepomorpha). Apollo Books. p. 38. ISBN 978-87-88757-78-1.
- ^ Hildebran, M.; Gonslow, G. (2001): Analysis of Vertebrate Structure. 5th edition. John Wiley & Sons, Inc. New York, page 33: Comment: The problem of naming sister groups
- ^ a b Benton, M.J. (1 November 2004). Vertebrate Palaeontology (Third ed.). Blackwell Publishing. pp. 33, 455 pp. ISBN 978-0632056378. Archived from the original on 19 October 2008. Retrieved 16 March 2006.
- ^ Irie, Naoki (26 December 2018). "The phylum Vertebrata: a case for zoological recognition". Zoological Letters. 4 Article Number 32: 32. doi:10.1186/s40851-018-0114-y. PMC 6307173. PMID 30607258.
- ^ Janvier, P. 1997. Vertebrata. Animals with backbones. Version 1 January 1997 (under construction). http://tolweb.org/Vertebrata/14829/1997.01.01 in The Tree of Life Web Project, http://tolweb.org/
- ^ Delsuc, F.; Philippe, H.; Tsagkogeorga, G.; Simion, P. (April 2018). "A phylogenomic framework and timescale for comparative studies of tunicates". BMC Biology. 16 (1). Tilak, M. K.; Turon, X.; López-Legentil, S.; Piette, J.; Lemaire, P.; Douzery, E. J.: 39. doi:10.1186/s12915-018-0499-2. PMC 5899321. PMID 29653534.
- ^ Friedman, Matt; Sallan, Lauren Cole (June 2012). "Five hundred million years of extinczion and recovery: A Phanerozoic survey of large-scale diversity patterns in fishes". Palaeontology. 55 (4): 707–742. Bibcode:2012Palgy..55..707F. doi:10.1111/j.1475-4983.2012.01165.x. S2CID 59423401.
- ^ Zhu, Min; Ahlberg, Per E.; Pan, Zhaohui; Zhu, Youan; Qiao, Tuo; Zhao, Wenjin; Jia, Liantao; Lu, Jing (21 October 2016). "A Silurian maxillate placoderm illuminates jaw evolution". Science. 354 (6310): 334–336. Bibcode:2016Sci...354..334Z. doi:10.1126/science.aah3764. PMID 27846567. S2CID 45922669.
- ^ Zhu, Min; Yu, Xiaobo; Ahlberg, Per Erik; Choo, Brian; Lu, Jing; Qiao, Tuo; Qu, Qingming; Zhao, Wenjin; Jia, Liantao; Blom, Henning; Zhu, You’an (25 September 2013). "A Silurian placoderm with osteichthyan-like marginal jaw bones". Nature. 502 (7470): 188–193. Bibcode:2013Natur.502..188Z. doi:10.1038/nature12617. ISSN 0028-0836. PMID 24067611. S2CID 4462506.
- ^
 Giles, Sam; Friedman, Matt; Brazeau, Martin D. (12 January 2015). "Osteichthyan-like cranial conditions in an Early Devonian stem gnathostome". Nature. 520 (7545): 82–85. Bibcode:2015Natur.520...82G. doi:10.1038/nature14065. ISSN 1476-4687. PMC 5536226. PMID 25581798.
Giles, Sam; Friedman, Matt; Brazeau, Martin D. (12 January 2015). "Osteichthyan-like cranial conditions in an Early Devonian stem gnathostome". Nature. 520 (7545): 82–85. Bibcode:2015Natur.520...82G. doi:10.1038/nature14065. ISSN 1476-4687. PMC 5536226. PMID 25581798.
- ^ Benton, Michael J. (2009). Vertebrate Palaeontology (3rd ed.). John Wiley & Sons. p. 44. ISBN 978-1-4051-4449-0.
- ^ Miyashita, Tetsuto; Coates, Michael I.; Farrar, Robert; Larson, Peter; Manning, Phillip L.; Wogelius, Roy A.; Edwards, Nicholas P.; Anné, Jennifer; Bergmann, Uwe; Palmer, A. Richard; Currie, Philip J. (5 February 2019). "Hagfish from the Cretaceous Tethys Sea and a reconciliation of the morphological–molecular conflict in early vertebrate phylogeny". Proceedings of the National Academy of Sciences of the United States of America. 116 (6): 2146–2151. Bibcode:2019PNAS..116.2146M. doi:10.1073/pnas.1814794116. ISSN 0027-8424. PMC 6369785. PMID 30670644.
- ^ a b c d The World Conservation Union. 2014. IUCN Red List of Threatened Species, 2014.3. Summary Statistics for Globally Threatened Species. Table 1: Numbers of threatened species by major groups of organisms (1996–2014).
- ^ Nelson, Joseph S. (2016). Fishes of the World. John Wiley & Sons, Inc. ISBN 978-1-118-34233-6.
- ^ Charlesworth, D.; Willis, J.H. (November 2009). "The genetics of inbreeding depression". Nat. Rev. Genet. 10 (11): 783–796. doi:10.1038/nrg2664. PMID 19834483. S2CID 771357.
- ^ Gallardo, J.A.; Neira, R. (July 2005). "Environmental dependence of inbreeding depression in cultured Coho salmon (Oncorhynchus kisutch): aggressiveness, dominance and intraspecific competition". Heredity (Edinb). 95 (6): 449–56. doi:10.1038/sj.hdy.6800741. PMID 16189545.
- ^ Ala-Honkola, O.; Uddström, A.; Pauli, B.D.; Lindström, K. (2009). "Strong inbreeding depression in male mating behaviour in a poeciliid fish". J. Evol. Biol. 22 (7): 1396–1406. doi:10.1111/j.1420-9101.2009.01765.x. PMID 19486236. S2CID 44317000.
- ^ Bickley, L.K.; Brown, A.R.; Hosken, D.J.; Hamilton, P.B.; Le Page, G.; Paull, G.C.; Owen, S.F.; Tyler, C.R. (February 2013). "Interactive effects of inbreeding and endocrine disruption on reproduction in a model laboratory fish". Evolutionary Applications. 6 (2): 279–289. Bibcode:2013EvApp...6..279B. doi:10.1111/j.1752-4571.2012.00288.x. PMC 3689353. PMID 23798977.
- ^ Ralls, K.; Ballou, J. (1982). "Effect of inbreeding on juvenile mortality in some small mammal species". Lab Anim. 16 (2): 159–66. doi:10.1258/002367782781110151. PMID 7043080.
- ^ Leroy, G. (August 2011). "Genetic diversity, inbreeding and breeding practices in dogs: results from pedigree analyses". Vet. J. 189 (2): 177–182. doi:10.1016/j.tvjl.2011.06.016. PMID 21737321.
- ^ van der Beek, S.; Nielen, A.L.; Schukken, Y.H.; Brascamp, E.W. (1999). "Evaluation of genetic, common-litter, and within-litter effects on preweaning mortality in a birth cohort of puppies". Am. J. Vet. Res. 60 (9): 1106–10. doi:10.2460/ajvr.1999.60.09.1106. PMID 10490080.
- ^ Gresky, C.; Hamann, H.; Distl, O. (2005). "[Influence of inbreeding on litter size and the proportion of stillborn puppies in dachshunds]". Berl. Munch. Tierarztl. Wochenschr. (in German). 118 (3–4): 134–9. PMID 15803761.
- ^ Leroy, G.; Phocas, F.; Hedan, B.; Verrier, E.; Rognon, X. (2015). "Inbreeding impact on litter size and survival in selected canine breeds" (PDF). Vet. J. 203 (1): 74–8. doi:10.1016/j.tvjl.2014.11.008. PMID 25475165. S2CID 27631883. Archived (PDF) from the original on 9 October 2022.
- ^ Keller, L. F.; Grant, P.R.; Grant, B.R.; Petren, K. (2002). "Environmental conditions affect the magnitude of inbreeding depression in survival of Darwin's finches". Evolution. 56 (6): 1229–39. doi:10.1111/j.0014-3820.2002.tb01434.x. PMID 12144022. S2CID 16206523.
- ^ Hemmings, N. L.; Slate, J.; Birkhead, T. R. (2012). "Inbreeding causes early death in a passerine bird". Nat Commun. 3: 863. Bibcode:2012NatCo...3..863H. doi:10.1038/ncomms1870. PMID 22643890.
- ^ a b Kingma, S. A.; Hall, M. L.; Peters, A. (2013). "Breeding synchronization facilitates extrapair mating for inbreeding avoidance". Behavioral Ecology. 24 (6): 1390–1397. doi:10.1093/beheco/art078. hdl:10.1093/beheco/art078.
- ^ a b Waldman, B.; Rice, J.E.; Honeycutt, R.L. (1992). "Kin recognition and incest avoidance in toads". Am. Zool. 32: 18–30. doi:10.1093/icb/32.1.18.
- ^ a b Fitzpatrick, J. L.; Evans, J. P. (2014). "Postcopulatory inbreeding avoidance in guppies" (PDF). J. Evol. Biol. 27 (12): 2585–94. doi:10.1111/jeb.12545. PMID 25387854. S2CID 934203. Archived (PDF) from the original on 9 October 2022.
- ^ a b Olsson, M.; Shine, R.; Madsen, T.; Gullberg, A. Tegelström H (1997). "Sperm choice by females". Trends Ecol. Evol. 12 (11): 445–6. Bibcode:1997TEcoE..12..445O. doi:10.1016/s0169-5347(97)85751-5. PMID 21238151.
- ^ Bernstein, H.; Byerly, H.C.; Hopf, F.A.; Michod, R.E. (1985). "Genetic damage, mutation, and the evolution of sex". Science. 229 (4719): 1277–81. Bibcode:1985Sci...229.1277B. doi:10.1126/science.3898363. PMID 3898363.
- ^ Pusey, A.; Wolf, M. (1996). "Inbreeding avoidance in animals". Trends Ecol. Evol. 11 (5): 201–6. Bibcode:1996TEcoE..11..201P. doi:10.1016/0169-5347(96)10028-8. PMID 21237809.
- ^ Szulkin, M.; Sheldon, B. C. (2008). "Dispersal as a means of inbreeding avoidance in a wild bird population". Proc. Biol. Sci. 275 (1635): 703–11. doi:10.1098/rspb.2007.0989. PMC 2596843. PMID 18211876.
- ^ Nelson-Flower, M. J.; Hockey, P. A.; O'Ryan, C.; Ridley, A. R. (2012). "Inbreeding avoidance mechanisms: dispersal dynamics in cooperatively breeding southern pied babblers". J Anim Ecol. 81 (4): 876–83. Bibcode:2012JAnEc..81..876N. doi:10.1111/j.1365-2656.2012.01983.x. PMID 22471769.
- ^ Riehl, C.; Stern, C. A. (2015). "How cooperatively breeding birds identify relatives and avoid incest: New insights into dispersal and kin recognition". BioEssays. 37 (12): 1303–8. doi:10.1002/bies.201500120. PMID 26577076. S2CID 205476732.
- ^ Booth, W.; Smith, C. F.; Eskridge, P. H.; Hoss, S. K.; Mendelson, J. R.; Schuett, G. W. (2012). "Facultative parthenogenesis discovered in wild vertebrates". Biol. Lett. 8 (6): 983–5. doi:10.1098/rsbl.2012.0666. PMC 3497136. PMID 22977071.
- ^ Booth, W.; Million, L.; Reynolds, R. G.; Burghardt, G. M.; Vargo, E. L.; Schal, C.; Tzika, A. C.; Schuett, G. W. (2011). "Consecutive virgin births in the new world boid snake, the Colombian rainbow Boa, Epicrates maurus". J. Hered. 102 (6): 759–63. doi:10.1093/jhered/esr080. PMID 21868391.
- ^ Bogart, J.P.; Bi, K.; Fu, J.; Noble, D.W.; Niedzwiecki, J. (February 2007). "Unisexual salamanders (genus Ambystoma) present a new reproductive mode for eukaryotes". Genome. 50 (2): 119–36. doi:10.1139/g06-152. PMID 17546077.
- ^ Bi, K.; Bogart, J. P. (April 2010). "Probing the meiotic mechanism of intergenomic exchanges by genomic in situ hybridization on lampbrush chromosomes of unisexual Ambystoma (Amphibia: Caudata)". Chromosome Res. 18 (3): 371–82. doi:10.1007/s10577-010-9121-3. PMID 20358399. S2CID 2015354.
- ^ Kanamori, Akira; Sugita, Yosuke; Yuasa, Yasufumi; Suzuki, Takamasa; Kawamura, Kouichi; Uno, Yoshinobu; Kamimura, Katsuyasu; Matsuda, Yoichi; Wilson, Catherine A; Amores, Angel; Postlethwait, John H (1 April 2016). "A Genetic Map for the Only Self-Fertilizing Vertebrate". G3: Genes, Genomes, Genetics. 6 (4): 1095–1106. doi:10.1534/g3.115.022699. ISSN 2160-1836. PMC 4825644. PMID 26865699.
- ^ Tatarenkov, A.; Lima, S. M.; Taylor, D. S.; Avise, J. C. (25 August 2009). "Long-term retention of self-fertilization in a fish clade". Proc. Natl. Acad. Sci. U.S.A. 106 (34): 14456–9. Bibcode:2009PNAS..10614456T. doi:10.1073/pnas.0907852106. PMC 2732792. PMID 19706532.
- ^ Sakakura, Yoshitaka; Soyano, Kiyoshi; Noakes, David L. G.; Hagiwara, Atsushi (2006). "Gonadal morphology in the self-fertilizing mangrove killifish, Kryptolebias marmoratus". Ichthyological Research. 53 (4): 427–430. Bibcode:2006IchtR..53..427S. doi:10.1007/s10228-006-0362-2. hdl:10069/35713. S2CID 9474211.
- ^ a b Avise, J. C.; Tatarenkov, A. (13 November 2012). "Allard's argument versus Baker's contention for the adaptive significance of selfing in a hermaphroditic fish". Proc. Natl. Acad. Sci. U.S.A. 109 (46): 18862–7. Bibcode:2012PNAS..10918862A. doi:10.1073/pnas.1217202109. PMC 3503157. PMID 23112206.
- ^ Earley, R. L.; Hanninen, A. F.; Fuller, A.; Garcia, M. J.; Lee, E. A. (2012). "Phenotypic plasticity and integration in the mangrove rivulus (Kryptolebias marmoratus): a prospectus". Integr. Comp. Biol. 52 (6): 814–27. doi:10.1093/icb/ics118. PMC 3501102. PMID 22990587.
- ^ "Living Planet Report 2018 | WWF". wwf.panda.org. Retrieved 21 May 2020.
- ^ a b Grooten, M.; Almond, R. E. A. (2018). Living Planet Report – 2018: Aiming Higher (PDF). WWF--World Wide Fund for Nature. ISBN 978-2-940529-90-2. Archived (PDF) from the original on 9 October 2022.
- ^ "WWF Finds Human Activity Is Decimating Wildlife Populations". Time. Retrieved 21 May 2020.
- ^ IPBES (25 November 2019). S. Diaz; J. Settele; E.S. Brondízio; H.T. Ngo; M. Guèze; J. Agard; A. Arneth; P. Balvanera; K.A. Brauman; S.H.M. Butchart; K.M.A. Chan; L.A. Garibaldi; K. Ichii; J. Liu; S.M. Subramanian; G.F. Midgley; P. Miloslavich; Z. Molnár; D. Obura; A. Pfaff; S. Polasky; A. Purvis; J. Razzaque; B. Reyers; R. Roy Chowdhury; Y.J. Shin; I.J. Visseren-Hamakers; K.J. Willis; C.N. Zay (eds.). Summary for policymakers of the global assessment report on biodiversity and ecosystem services. Bonn: IPBES Secretariat. pp. 1–56. doi:10.5281/zenodo.3553579.
Bibliography
[edit]- Kardong, Kenneth V. (1998). Vertebrates: Comparative Anatomy, Function, Evolution (second ed.). USA: McGraw-Hill. pp. 747 pp. ISBN 978-0-697-28654-3.
- "Vertebrata". Integrated Taxonomic Information System. Retrieved 6 August 2007.
External links
[edit]- Tree of Life
- Tunicates and not cephalochordates are the closest living relatives of vertebrates
- Vertebrate Pests chapter in United States Environmental Protection Agency and University of Florida/Institute of Food and Agricultural Sciences National Public Health Pesticide Applicator Training Manual
- The Vertebrates
- The Origin of Vertebrates Marc W. Kirschner, iBioSeminars, 2008.

